It is the carbon source for plants. A carbon sink is any reservoir natural or otherwise that accumulates and stores some carbon-containing chemical compound for an indefinite period and thereby lowers the concentration of carbon dioxide CO 2 from the atmosphere.

Electrochemical Capture And Storage Of Co2 As Calcium Carbonate Oloye 2021 Chemsuschem Wiley Online Library
:max_bytes(150000):strip_icc()/__opt__aboutcom__coeus__resources__content_migration__treehugger__images__2017__05__making-graphene-by-CVD-7d97f5f253664f6bb9637a1f49ad05bb.png)
Graphene Infused Lime Paint Has Magical Green Properties

Reducing Emissions From Cement Steel Production Cleantechnica
Students are introduced to the concept of energy cycles by learning about the carbon cycle.

Limestone hydrogen absorb co2. The underlying idea is that trees constantly absorb and store CO2. Absorb Cl ions from the evolved gas. Cement use is set to rise as global urbanisation and economic development increases demand for new buildings and infrastructure.
Absorb moisture from the gas D. Ullmanns Encyclopedia of Industrial Chemistry. This is a green renewable energy that is generating power 247 unlike wind and solar.
These solutions are produced industrially by dissolution of hydrogen bromide in water. When CO2 dissolves in water it makes carbonic acid. These changes can be accelerated through changes in land use and agricultural.
The lime would be made by heating limestone calcium carbonate a well-known industrial process. Federal Republic of Germany. - temperature increased followed by an increase in CO2.
The CO2 transfers the heat 5-10 times better than water and the superheated gas formed by the CO2 heat can be used to drive an electric turbine. To be stocked with solvent to absorb CO2. It plays a crucial role in the weathering of rocks.
QuestionWhen hydrogen chloride gas is prepared on a humid day the gas is usually passed through the guard tube containing calcium chloride. The lime seizes hold of the dissolved CO2 producing small flakes of limestone. With more greenhouse gases in the air heat passing through on its way out of the atmosphere is more likely to be stopped.
The product of that reaction calcium carbonate is then deposited onto the ocean floor where it becomes limestone. In 2015 it generated around 28bn tonnes of CO2 equivalent to 8 of the global total a greater share than any country other than China or the US. While thats going on some of the carbon monoxide is transformed into carbon dioxide.
Calcium oxide added to the ocean. - several times in the past humans have perturbed the natural environment causing natural increases in CO2 and temperature. The gas mostly contains CO2 hydrogen H2 and carbon monoxide CO.
They learn how carbon atoms travel through the geological ancient carbon cycle and the biologicalphysical carbon cycle. Constant-boiling hydrobromic acid distills at 1243 C at atmospheric pressure and contains 4763 wt hydrogen bromide. Non-dispersive infrared cells are based on the principle that CO2 and SO2 absorb infrared IR energy at unique wavelengths within the IR spectrum.
Hydriodic acid is the colorless solution formed when hydrogen iodide gas dissolves in water and at commercial strength typically contains 47 hydrogen iodide. Absorb the evolved gas B. I have big doubts that this can happen to provide peak power.
Carbon dioxide CO2 is naturally captured from the atmosphere through biological chemical and physical processes. Plumes of carbon dioxide in the simulation swirl and shift as winds disperse the. NASA A Year in the Life of Earths CO2.
Oceans have a large capacity to absorb CO 2 thus reducing the amount of CO 2 in the atmosphere and bringing carbon atoms into the ocean system. It will not get us to net zero but it may help reduce CO2 emissions. Carbon dioxide is an atmospheric constituent that plays several vital roles in the environment.
Incident IR energy at these wavelengths is absorbed as the gases pass through IR absorption cells with the absorption being. They consider how human activities disturb the carbon cycle by emitting carbon dioxide into the atmosphere. They discuss how engineers and scientists are working to reduce.
Use renewable energy to produce hydrogen which can be stored energy. Adding lime to the sea would increase its capacity to absorb CO 2. Distilled water can contain CO2 which can produce H2OCO2-H2CO3-H2OCO2 depending which system of distillation is used for.
As cement is made from limestone it releases carbon dioxide. -- Using MHHOOhmasa Gas as a fuel Cements production cost will be very low - about 13 of the acual market price. Photograph 2009 Greg Carley.
The boiling point and hydrogen bromide concentration can be partially controlled by varying the pressure during distillation. In the ocean the calcium ions combine with bicarbonate ions to form calcium carbonate the active ingredient in antacids and the chalky white substance that dries on your faucet if you live in an area with hard water. The vast amounts of limestone in the earths crust are a result of carbon.
The first is the chemical reaction involved in the production of the main component of cement clinker as carbonates largely limestone CaCO3 are decomposed into oxides largely lime CaO and CO2 by the addition of heat. When hydrogen chloride gas is prepared on a humid day the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to A.
An ultra-high-resolution NASA computer model has given scientists a stunning new look at how carbon dioxide in the atmosphere travels around the globe. It is stored in biomass organic matter in sediments and in carbonate rocks like. Global warming is expected to reduce the oceans ability to absorb CO 2 leaving more in the atmospherewhich will lead to even higher temperatures.
Moisten the gas C. MHHO clean fuel 1. It is a greenhouse gas that traps infrared radiation heat in the atmosphere.
Later a separate reaction converts water H2O into hydrogen. Oceans and the Carbon Cycle Part A. CO2 can be injected into thermal rock which is hot.
Wiley-VCH Verlag GmbH Co. That is why pH value of simple distilled water is lower than 70. However some of the carbon atoms from.
- temperatures and CO2 concentration have remained constant until very recent inputs from humans. Graph by Robert Simmon In the short term the ocean absorbs atmospheric carbon dioxide into the mixed layer a thin layer of water with nearly uniform temperature salinity and dissolved gases. Public awareness of the significance of CO 2 sinks has grown since passage of the 1997.
Producing hydrogen on a large scale is like storing energy. Carbon sequestration is the long-term removal capture or sequestration of carbon dioxide from the atmosphere to slow or reverse atmospheric CO 2 pollution and to mitigate or reverse climate change. The added greenhouse gases absorb the heat.
Coal oil or natural gas is heated in steam and oxygen resulting in a synthesis gas or syngas. They then radiate this heat. But some compounds.
Many CO 2 molecules that diffuse into sea surface waters diffuse back to the atmosphere on very short time scales. Stoichiometry directly indicates how much CO2 is. Globally the two most important carbon sinks are vegetation and the ocean.
Farm animals for example release methane gas as they digest food. 2003 to Present p. This is a form of enhanced geothermal energy EGS.
If the cement industry were a country it would be the third largest emitter in the world. The role of calcium chloride taken in the guard tube is to a absorb the evolved gas b moisten the gas c absorb moisture from the gas d absorb Cl ions from the evolved gas 92. Down to the Deep - The Oceans Biological Pump.

Hydrogen Production With Co2 Capture Sciencedirect

Slaked Lime Injection In The Upwelling Currents Download Scientific Diagram

Carbon Sink Wikipedia

Petrifying Climate Change Hakai Magazine

Integration Of Thermochemical Water Splitting With Co2 Direct Air Capture Pnas

Hydrogen Production With Co2 Capture Sciencedirect
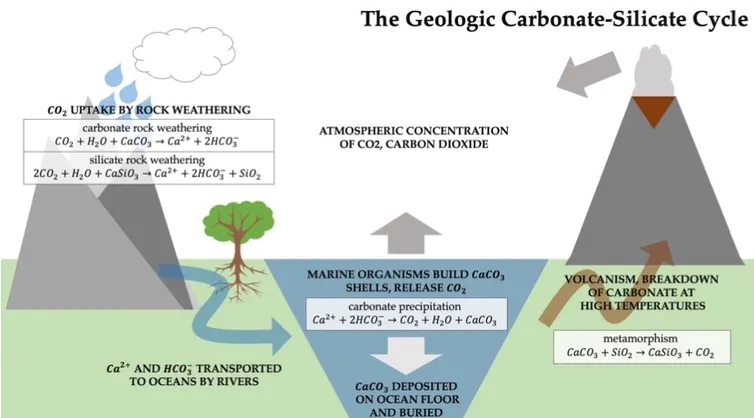
Enhanced Weathering Crushed Rocks Spread On Farmland Can Capture Billions Of Tons Of Co2 Year Energy Post

Water Gas Shift Reaction For Hydrogen Production And Carbon Dioxide Capture A Review Sciencedirect
